The first of its kind, Zolgensma, received FDA approval for a single dose administered therapy that uses the protein capsule of adeno-virus with double stranded complementary gene therapy implanted inside, intended to cure spinal muscular atrophy type 1, a genetic disease with a 95% rate of mortality by 18 months of age for infants.
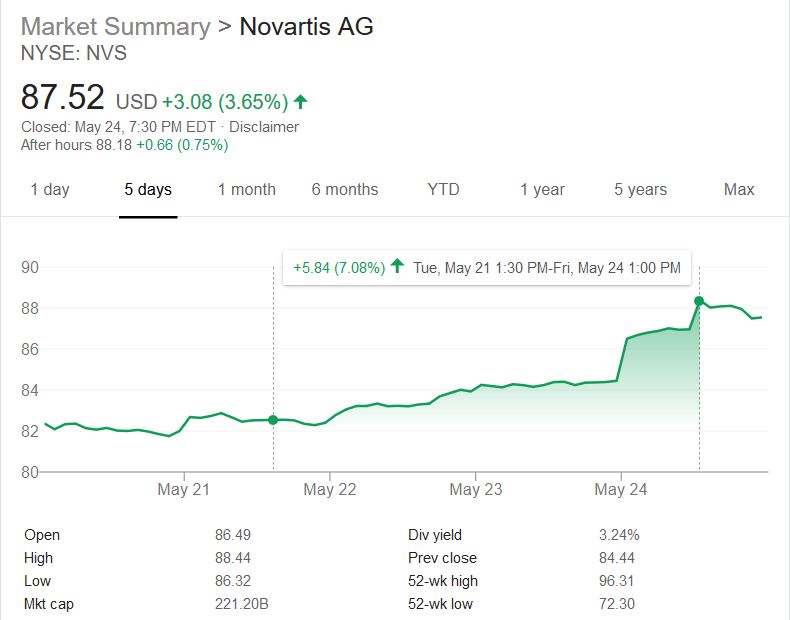
It sold by Novartis and costs $2.125 million USD. This potential 1 billion dollar blockbuster drug shakes the world as a first step to life saving curative precision medications being approved to save those with genetic diseases. Is the cost justified? Will Novartis’s new potential earnings sway more money in the future to the pockets of investors and c-suite holders or more lifesaving pipeline R&D?
What Drug: Zolgensma (AVXS-101), a biological gene therapy with the ability to only need one administration per lifetime.
Who Made It: Developed by AveXis and owned by Novartis.
Cost: $2,125,000.00 one time cost with the ability to be paid in $425,000.00 increments over 5 years
Why This Cost: Spinraza (nusinersen), the competition to Zolgensma (AVXS-101), costs $375,000 a year, but has a downside of needing to be administered 3 times a year intrathecally (injected to the spinal cord). The innovation of a onetime life-saving treatment essentially is adding a 13.33% ($50,000) premium to the cost.
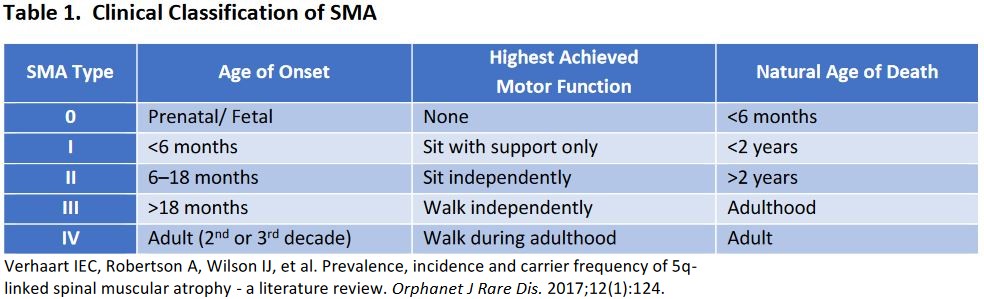
Who Will This Treat: Infants born with spinal muscular atrophy (SMA) type 1. Those with SMA type 1 have severe, progressive muscle weakness and flaccid or reduced muscle tone and eventually pass away from complications by 18 months of age. A mutation in the survival motor neuron gene 1 (SMN1) leads to a lower production in the SMN protein. SMN2, another gene, is capable of producing the SMN protein but with a max length of 10%.
How It Works In Short: A virus shell is used to deliver a set of genes that let the patient produce the SMN protein they lack.

The outside of adenovirus, which is what is used to deliver Zolgensma.
Diving Deeper: Zolgensma (AVXS-101) uses a non-copying virus protein shell, known as a capsid, to cross the blood brain barrier to deliver a fully functional complementary copy of the human SMN gene to the patient with an amazing feature of being always promoted, in the production-turned-on mode, thanks to a special section (beta-actin) being added on from a cytomegalovirus enhanced chicken hybrid.
Discussion: There is no doubt in my mind that this medication is amazing step towards a future with genetic diseases and cancer being on a level playing field. The capsid and double stranded technology AveXis uses is an amazing mode of action that will put competition on oligopeptide antisense technology and even CRISPR Cas-9 applications. The clinical trials shows an amazing level of success with these infants that would have a 95% chance of death by 18 months of age But at what point do we start talking about medications in regards to economy (epinephrine), sports (Eliquis), luxury (Chimera), or hyper (Zolgensma) class tier?
I must admit it is strange to think of a price of life saving medication being equated to a display of wealth and luxury. In our health system we already have bronze, silver, gold, and platinum healthcare plans that have different prescription coverage tiers, generally 3-4, to determine your medication out of pocket cost. The price of an ampule of epinephrine, ~$2.00, can save a life with one injection too (sometimes more). Yet, Mylan can sell a delivery system of epinephrine costing at one point $600-$700 dollars. Would you like the economy cloth or Italian leather? I have mixed feelings when I say at least there is an alternative medication to Zolgensma called Spinraza. Competition is normally a great so you can have choices. Your choices are a lifetime annual cost of $375k vs a one time cost of $2.125 million. Of note, Spinraza is given intrathecally every 4 months and is more preservation instead of a potential cure. What decisions, legal implications, and options would a family have if they choose the 5 year payment plan and no long have that insurance due to unemployment? On the other hand, this is an extreme accumulation of hours, intelligence, and funds that will save hundreds of lives. The past diagnosis and outcomes of SMA projects many deaths that would be at hand in the future if this medication did not exist. It is a blessing to have two solutions.
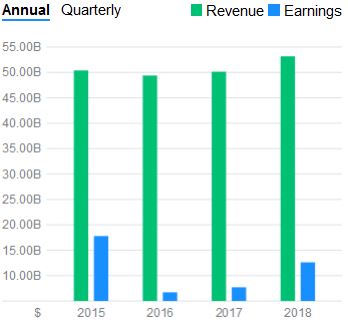
I decided to crunch the math (450 to 500 (Let’s choose 475) SMD Type 1 child births per year x $2.125 million USD = $1.009 billion potential in sales per year). It was evident that this medication had an amazing potential in the amount of gained revenues for the company. In context, the #1 stop belongs to Abbvie who sold over $19.936 billion’s worth of Humira and #10 belonging to Bayer J&J’s $6.589 billion’s worth of Xarelto sales . Both of these medications improve the quality of lives of people and prevent deaths but neither have the feature of a one time administration. Additionally, the potential extra ~$1 billion per year would lead to only a 2% increase in revenue per year for Novartis. One could see the company’s asking price is close to the rate of inflation and is trading its curative lifesaving product for a 2% increase in growth. I wish the scale was not an average of $50 billion dollars but, it was a risk, they will regain, and continue with this money to make even more amazing life saving advancements. It is nice to see money being spent on researching to cure rather and coming out with a product instead of a new more efficient way of war.
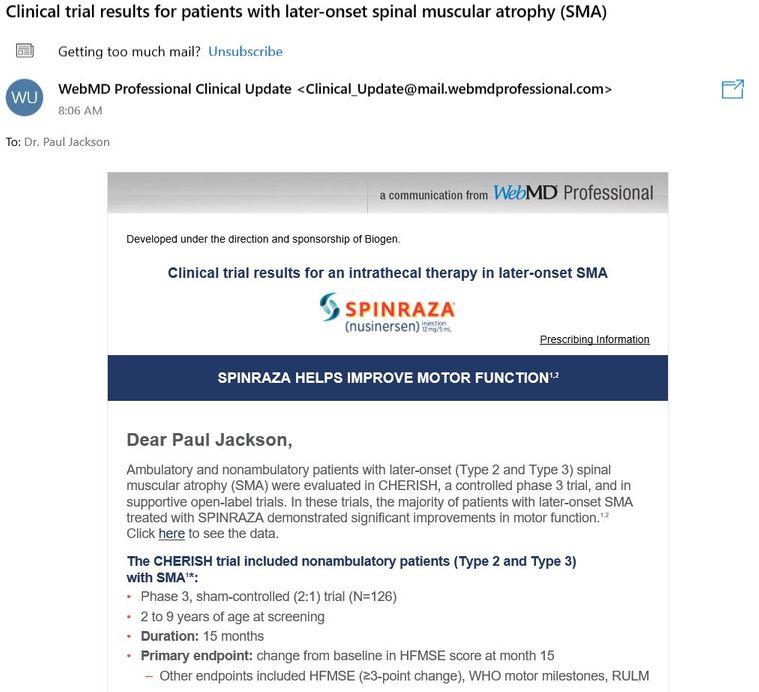
There is good news that some might not be aware of. A population of those with SMA type II-IV who have incredible challenges they face daily have a chance to be recruited into a new trial for Zolgensma. It is for SMA patients from 6 months to 60 months of age at the current moment. The differentiating factor is the fact Zolgensma will be given intrathecally instead of IV; allowing it to be used on more than just infants. I can only imagine what it would be like to hear this information if I was affected by SMA! It must be great but, with a big gulp of financial questions. Click here to read more about the trial.
As always we here at DrPGx are always keeping up-to-date with the latest and greatest findings related to precision medicine, genetics, nutrition, and pharmacotherapy. If you ever need to sit down and talk about research to what is on the horizon, make sure to book a session with us! As always, God Bless!
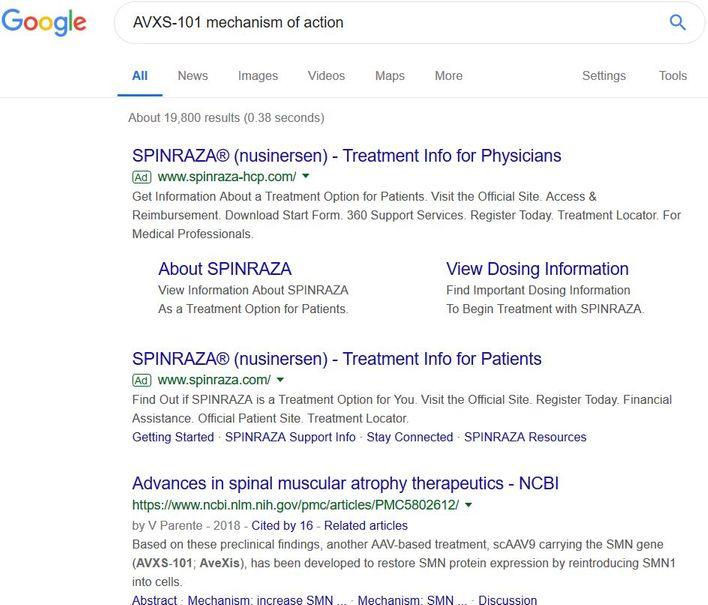
5/26/2019 Update: Upon receiving a “Professional Clinical Update” this morning I was interested to learn and see that the topic was about the CHERISH trial, which uses Spinraza for the treatment of Type II/III SMA. It is always interesting to see how pharmaceutical companies run their ad campaigns. Even when I did a quick google search, I was interested to find out that Spinraza was the first two ads with even a less asked, “AVXS-101 mechanism of action” search. When you have billions at stake, I can only imagine how involved and motivated both marketing teams are at this point.



0 Comments