CRISPR Cas-9 cuts DNA at specific places so that bits of DNA can be added or removed. This method of genome editing is used for potetially curing several diseases such as TDT.
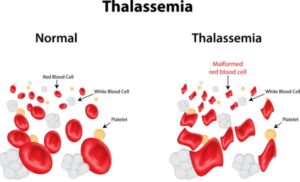
 Vertex Pharmaceuticals Incorporated, a very interesting company in itself, is embarking as the first company to start a Phase 1/2 clinical trial and infuse a patient with their investigational drug therapy as of writing this article. Vertex is using the CRISPR/Cas9 gene-editing therapy for the treatment that could potentially cure transfusion-dependent beta thalassemia, known as TDT. TDT is a form of an inherited blood disorder that requires blood transfusion due to mutations in the beta-globin gene that cause the red blood cells to become stiff and sickle in shape. This in turn can shorten the lifespan of those affected by TDT due to iron accumulation, organ damage, and small vessel blockage.
Vertex Pharmaceuticals Incorporated, a very interesting company in itself, is embarking as the first company to start a Phase 1/2 clinical trial and infuse a patient with their investigational drug therapy as of writing this article. Vertex is using the CRISPR/Cas9 gene-editing therapy for the treatment that could potentially cure transfusion-dependent beta thalassemia, known as TDT. TDT is a form of an inherited blood disorder that requires blood transfusion due to mutations in the beta-globin gene that cause the red blood cells to become stiff and sickle in shape. This in turn can shorten the lifespan of those affected by TDT due to iron accumulation, organ damage, and small vessel blockage.
The FDA granted Vertex Fast Track Designation for the treatment of SCD in January 2019. The method of the trial is of interest. Vertex is using CTX001 (The investigational name of the therapy) to treat stem cells collected from peripheral blood, infuse the hematopoietic stem cells back, and monitor to see if the the edited cells begin to produce mature blood cells, and measure outcomes . The outcomes of this study will be extremely important due to the off-target issues that plagues CRISPR/Cas9 therapy. In theory some off-target changes could be beneficial, neutral, or detrimental. Time, separate trials, and a large number of people tested will help the scientific, regulatory committees, and general public who carry or present with genetic disease, will determine how to proceed with such a powerful tools found in the genetic codes of old prokaryotic organisms.
A question to consider with CRISPR/Cas9 therapy is how quickly can these treatments can commercially start to be used in a safe and effective manner to help save lives, ease pain, and stop suffering. Will oral antisense oligonucleotides be the way of the future for genetic diseases? Time will tell.
.




0 Comments